
The following is a transcript of an interview by Oriana Kraft with Priyanka Jain, Co-Founder and CEO of Evvy, an at home vaginal microbiome test designed to help get to the root of recurrent vaginal infections and other symptoms.
This is Part 4 of a series of Deep Dives on Sex Specific Topics in Medicine, in the aim of connecting students with research being done in the field.
Hi Priyanka! Can you tell me a little bit about your background and how it is you came to work in the field of women’s health?
I come at this from the data science and AI side. My life-long interest has been in making life better for women across the globe. I started in the non-profit sector and worked with the UN to launch the ‘Girl Up’ campaign. I’ve done a lot of work around women’s access to education and healthcare. But it’s only when I started to look at the percolation of data into the healthcare system, that I really began to notice how the algorithms we use don’t take into account all the ways in which the female body might be different.
On a personal level, I became curious about how we could use data to make informed decisions about women’s health. In particular, I wanted to investigate which biomarkers of health are sitting in the female body unused – biomarkers that we never tracked or measured because we never studied women. There are many things that the female body does uniquely that could be incredible measurements of women’s health. These biomarkers could help us predict diagnose, treat or stratify the risk of disease as it manifests differently in the female body. The problem is we don’t typically use any of those biomarkers because they don’t exist in men so we never studied them.
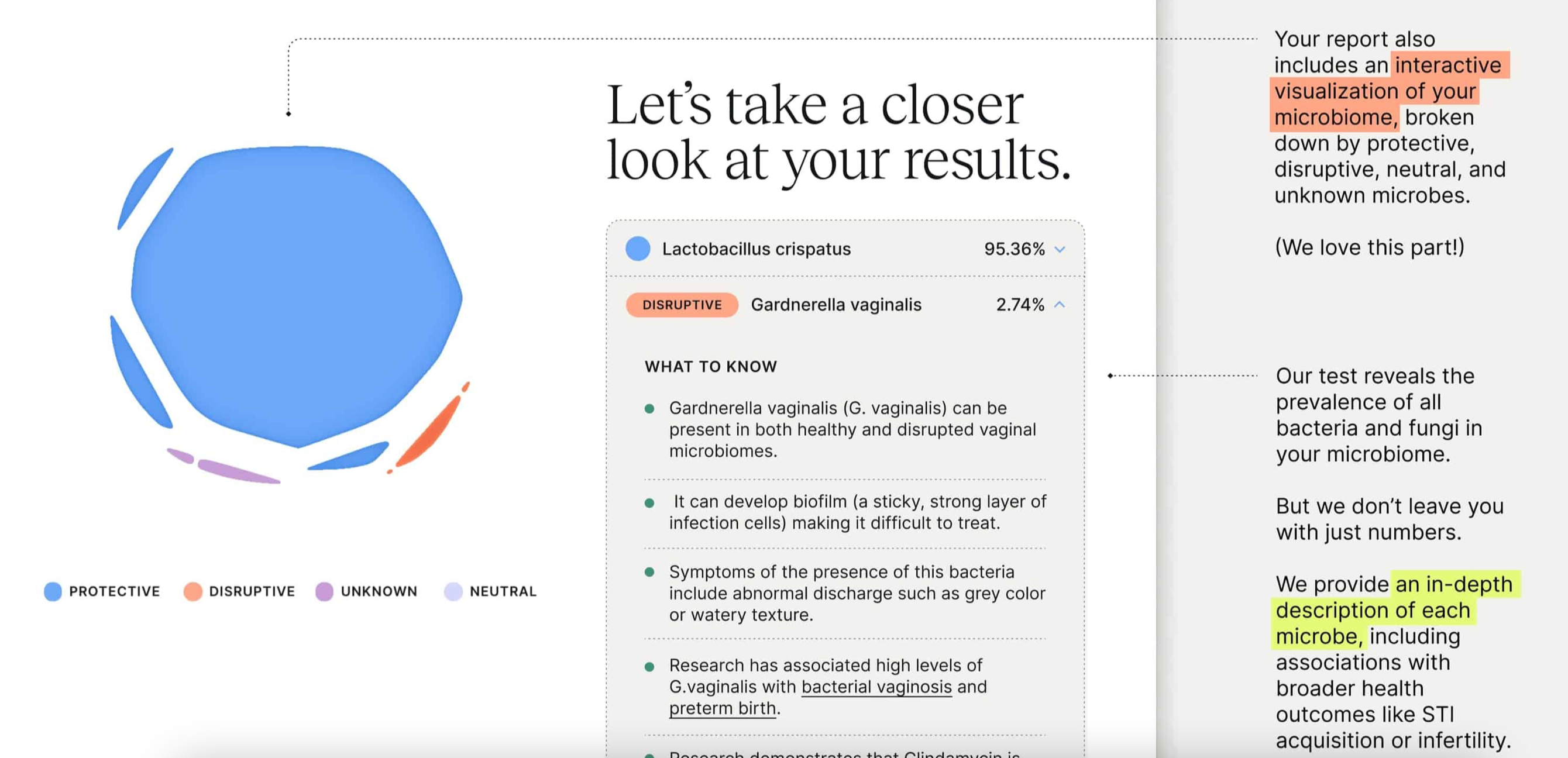 How did you settle on the idea of the vaginal microbiome?
How did you settle on the idea of the vaginal microbiome?
I interviewed 200 researchers, scientists, doctors, women, investors, founders and I just kept coming back to the vaginal microbiome as an area that was in desperate need of innovation – which was shocking to me because I didn’t even know the vaginal microbiome was a term. And yet, it turns out the vaginal microbiome is the cause of a lot of the most common reproductive health issues women face. The number one reason women go to the OBGYN are vaginal infections: yeast infections, UTIs, bacterial vaginosis. It’s incredibly prevalent. Simultaneously, I couldn’t believe the amount of research coming out saying the composition of the vaginal microbiome is associated to everything from fertility challenges, to cervical cancer, to preterm birth, to STI acquisition. It made me wonder why I don’t have access to that type of information about my own body and, more importantly, why no one in the healthcare system was using this data to make better decisions about my health.
It just became clear to me that the vaginal microbiome was the most obvious starting point to make a dent in women’s healthcare experience. Consumers want to be able to access the information because of the vaginal microbiome’s link to UTIs and yeast infections. And the vaginal microbiome also provides value on the provider side because of its link to some of the most expensive health outcomes for women.
How do you see Evvy as being a part of helping bridge the gender data gap?
I firmly believe that women can be a part of our own solution. We can move towards bridging the data gap by giving consumers access to this type of information about themselves. That’s how it becomes common place to have this sort of information in the first place.
Are you finding that women understand the value of testing their vaginal microbiome?
Definitely. Recurrent UTIs, yeast infections, STDs – these are all things women have struggled with for so long but there’s never been a brand or a movement attached to it before. There are so many women who have been dying for answers, living in these Facebook communities where it’s the blind leading the blind and there’s a lack of answers. Now that they have the possibility to get real answers they definitely understand the value of being able to test themselves.
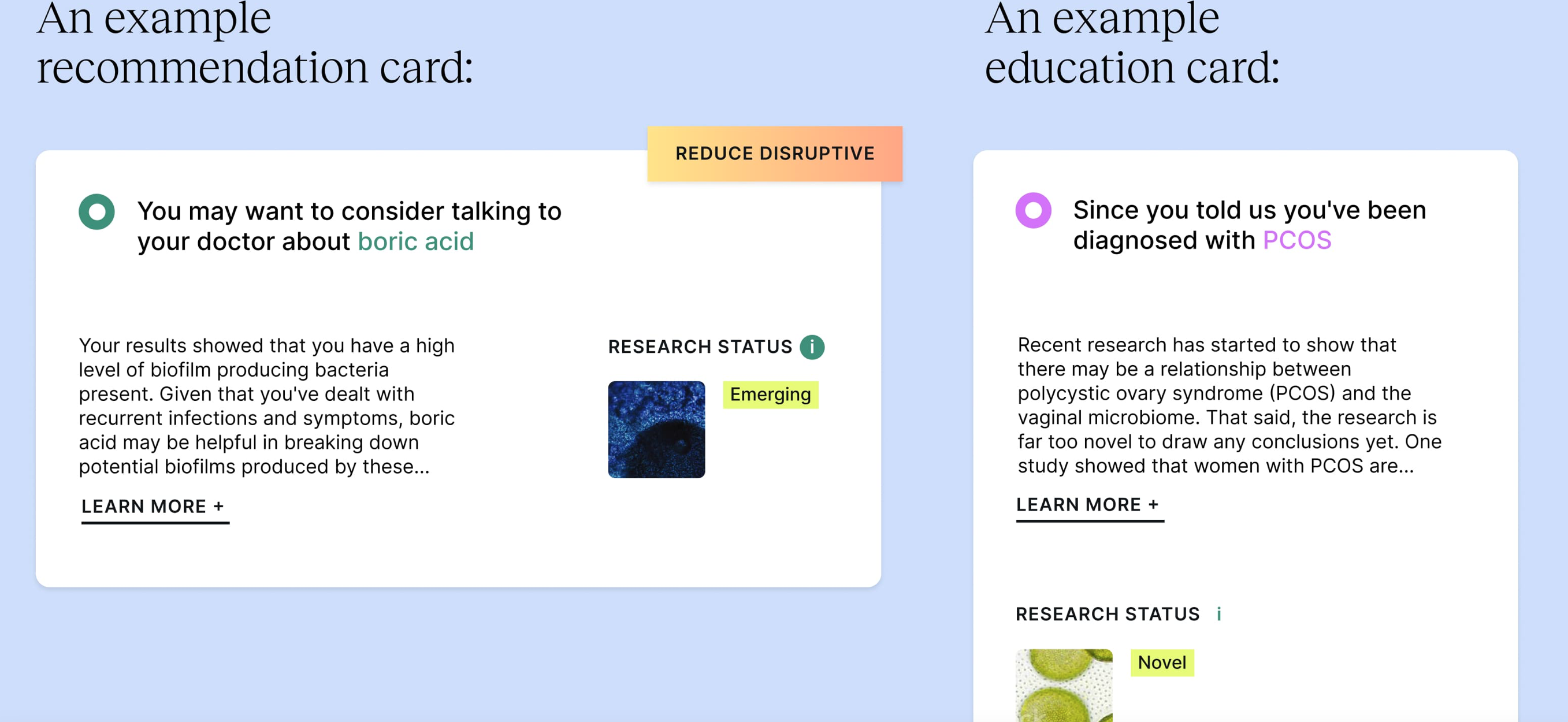 And Evvy gives women actionable insights to work off, right? How does that work?
And Evvy gives women actionable insights to work off, right? How does that work?
Most of our customers take the vaginal microbiome test every three months. They track what they do in-between for themselves. They track things like whether they had a new sexual partner between tests, whether they tried out a new diet, used a new probiotic. At Evvy, we’re able to show them; ‘you started here’, ‘this is what you did in between’, ‘this is where your vaginal microbiome is now’. We can also then show other users what worked and didn’t work for users who started out in a similar place to them.
What sort of information can Evvy provide to women going through menopause?
One of the main things that happens in menopause is a decline in estrogen. As estrogen goes down so does glycogen. Glycogen is what feeds a lot of the protective bacteria in the vaginal microbiome. As a lot of women enter menopause they get vaginal infections for the first time because they’re losing a lot of the healthy bacteria in their vaginal microbiome. At Evvy, we can help women suffering from vaginal infections during menopause to understand whether the reason they are having an increase in vaginal infections is that the number of their lactobacilli is going down and if it is how they can promote an increase in the quantity of lactobacilli they have.
_________________________________________________________
Innovations like Evvy’s at home vaginal microbiome test is why we need Femtech. (And a FemTech Summit like this one of course). Want to hear more about how the vaginal microbiome could be the key to cracking women’s health? Sign up for the 2022 femtech conference here.
__________________________________________________________________________________________________________________
 The following is a transcript of an interview by Oriana Kraft with Mette Dyhrberg, the founder of Mymee, a data-powered digital care program for people with autoimmunity and COVID long haul.
The following is a transcript of an interview by Oriana Kraft with Mette Dyhrberg, the founder of Mymee, a data-powered digital care program for people with autoimmunity and COVID long haul.
Could you give me some background on Mymee and the work you do?
I’m the founder of Mymee a digital coaching program for people with autoimmune disorders. Autoimmune diseases have a prevalence that skews over eighty percent female. It’s an issue that impacted one in four hundred people in our parents’ generation but now impacts one in five people instead. Today autoimmune diseases are the leading cause of death in women between fifteen to forty-five years old.
And how do you use data to improve health outcomes at Mymee?
Mymee is a data driven program. The data is the driver of any intervention that people are being given. Studies have shown that 80% of the immune system is determined by lifestyle and the environment. At a high level, our program collects and analyzes self-reported data about these different variables using a simple app to create a highly personalized health plan. The app is custom-tailored to each individual so whether you’re recording what food you ate, reporting your symptoms or tracking a runny nose – self-reporting becomes very easy. We take this body signaling and turn that noise into understanding by pinpointing the causalities between what you do and how it impacts your symptoms. Mymee’s approach is led by independently certified health coaches that have all been trained on how to use Mymee’s data platform to turn machine insights into a personalized plan. Our coaches are able to help you successfully pinpoint and then avoid your triggers through small doable changes made over time.
Could you give me some examples of what triggers could be?
Eighty-two percent of triggers are dietary. The remainder is environmental and lifestyle factors like toxins and stress. To date we have reversed symptoms in sixty-seven autoimmune diseases based on our protocol. When COVID came about it became very clear to us that for some people COVID acted as an accelerator of autoimmunity, resulting in such familiar, persistent symptoms like fatigue, brain fog, joint pain and aches.
Could you elaborate a bit on your work with COVID?
Essentially, we use our methodology to jump start the recovery process for COVID long haul. One of the things that’s come to light in our work with COVID long haulers since June 2020 is the role real-world evidence can have in targeting and reducing autoimmunity-related symptoms. Interventions based on real-world evidence can deliver immediate benefit and we’ve seen some great success using this approach to guide interventions based on finding personal food and lifestyle triggers. For example, we noticed that protein intake impacted the severity of symptoms for a lot of patients. As a trusted performance partner of Mount Sinai, we’re also working with patients at their post-COVID Recovery Clinic to help with recovery.
Do you believe the fact that autoimmune disorders primarily impact women has had an impact on the lack of innovation in curing autoimmune disorders?
Unfortunately, I do believe that it is very much linked. I’m astounded that we could have diseases in the US go from impacting one in four hundred people to one in five and have no alarm bells go off. We are in a position where women are being told it’s all in their head for five to seven years before they receive a diagnosis. Even after they receive a diagnosis, medication fails for three out of four people. And it’s not just the women we are failing. We are failing their children also. Autoimmune disease costs our society a lot more than cancer does, yet the research dollars allocated to autoimmune diseases account for only a sliver of the research dollars dedicated to cancer. You can’t tell me that has nothing to do with the demographic break-down of autoimmune disorders.
How do you believe we can start to personalize women’s health?
Well, a nice place to start would be to test drugs on women as well as men. The fact that metformin, which is very loosely given to treat insulin disorders, has only been tested in men is outrageous to me. There are so many pockets of healthcare where personalization has completely missed the boat. To be quite frank as a society we have often not thought about health in a full picture way. If you have an autoimmune disease, it gets taken apart. You go to the doctor and have brain fog, stomach pain, joint issues – and the doctor tells you: “That’s three different specialists. What’s your biggest problem right now?” At that point, we fail the patient. Joints are not a separate issue from the brain fog or from stomach pain. It’s all interrelated. When women seek help for fertility issues that’s a pre-autoimmune problem. You are in a position where your body is not working and these are the first signs. As a society we just figure out how to manipulate the body to actually give birth instead of taking a look at the underlying issues. Infertility is inflammatory driven. Autoimmunity is inflammatory driven. Many issues are driven by the same underlying cause but we separate them into six different buckets and say they all have different treatment methodologies.
How do you think we can get a more holistic picture of patients?
By companies like ours going directly to the consumer in need. There are enough women out there that know there is something wrong with the treatment they are being given. I think it is going to be a grassroots movement. I think doctors will be referring in. It has to be an interplay between the disruptive methodologies, the data and the doctors – but it will begin with women themselves. We’ve never had customer service in healthcare be as good as if you were buying a pair of sneakers.
Do you find doctors are responsive to Mymee?
When the data started backing what we do, doctors became very responsive. We’re an adjunct therapy to the care doctors deliver after all. We are a tool for the rheumatologist, the same way the electric toothbrush is a tool for the dentist. Whatever it is doctors need, we help them provide that. I believe doctors understand how a data-driven solution can be helpful, but they’ve had limited experience seeing how effective the type of personalized interventions we suggest can be until they have patients come back with exponentially improved symptoms. We are giving people the blueprint to their own bodies.
______________
Innovations like Mymee’s data tracking to understand autoimmune diseases are why we need Femtech. (And a FemTech Summit like this one of course). Want to hear more about how data could be the key to long-term recovery from autoimmune diseases? Sign up for the 2022 femtech conference here.
____

What Can A Smart Vibrator Tell Us About The Link Between Women’s Sexual Pleasure And Women’s Health?
The following is a transcript of an interview by Oriana Kraft with Anna Lee, Co-Founder of Lioness and Head of Engineering. This is Part 3 of a series of Deep Dives on Sex Specific Topics in Medicine, in the aim of connecting students with research being done in the field.
What does Lioness do?
We’re a women led female sexual wellness company. We built the first and only smart vibrator that helps people learn about their bodies through biofeedback data. We measure pelvic floor movements which is a great indicator of arousal and orgasms. People can use it like a normal, rabbit-style vibrator while the sensors and technology work in the background. Through the mobile app, we visualize the data to show them what’s working, what’s changing and how different external factors could be affecting pleasure and arousal. Our app allows you to track the impact of environmental changes—everything from caffeine to sleep quality. Via Bluetooth we’re also able to chart a user’s session or orgasm in real time.
Do you feel people inherently understand what the implications of having that data is?
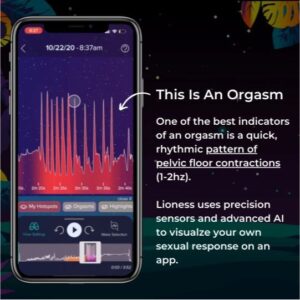 Being able to visualize what the data looks like helps. With Lioness you can visualize what arousal looks like normally and compare it to what it looks like when you don’t get enough sleep or when you’re stressed. Being able to see that gets people in the mindset of how they can see arousal as a quantified data point versus arousal ‘just being a feeling’. In sex therapy, the biggest recommendation they’ll give you is to keep a sex log of what’s working for you and what’s not so you can be mindful of what changed and what impact external factors had on your experience. We try to piggy-back off that concept by adding biofeedback. Sexual function is just like eating, breathing and sleeping after all. In our day and age, it’s not that uncommon to track what you eat and how you sleep. So, why wouldn’t you track something as intimately a part of your life as sexual function to see if you can improve it?
Being able to visualize what the data looks like helps. With Lioness you can visualize what arousal looks like normally and compare it to what it looks like when you don’t get enough sleep or when you’re stressed. Being able to see that gets people in the mindset of how they can see arousal as a quantified data point versus arousal ‘just being a feeling’. In sex therapy, the biggest recommendation they’ll give you is to keep a sex log of what’s working for you and what’s not so you can be mindful of what changed and what impact external factors had on your experience. We try to piggy-back off that concept by adding biofeedback. Sexual function is just like eating, breathing and sleeping after all. In our day and age, it’s not that uncommon to track what you eat and how you sleep. So, why wouldn’t you track something as intimately a part of your life as sexual function to see if you can improve it?
And you collaborate with researchers surrounding the data you gather, right?
Yes, that’s right. We have the world’s largest data set in terms of female physiological sexual function. Which brings up a whole set of possibilities. What can you do with a dataset like that when you take out the barrier of there not being enough researchers or funding? If you think about it, in terms of men’s health there has been billions of dollars pumped into researching men’s sexual pleasure.
We know so much about sexual health and how that’s connected to men’s well-being but that research hasn’t really been done when it comes to women. It’s a very outdated notion that there are still so many unknowns regarding women’s sexual pleasure. We know a lot of the causes of erectile dysfunction for example. We know that erectile dysfunction can be caused by underlying conditions like diabetes, excessive smoking, heart disease – so wouldn’t it be interesting to examine whether there are underlying health conditions when women have difficulty orgasming ? Anecdotally, we had a user, an athlete, write to us saying: ‘Hey, I think something’s wrong with your vibrator it hasn’t been working these past few days’. We looked at her data then and we saw that the last two days when she used Lioness the biofeedback line was flat. We got back to her and asked if something happened to her two days ago. She thought about it and it turned out that two days ago she had a concussion. It’s examining links like that.
______________________________________________________________________________________________________________________

The following is a transcript of an interview by Oriana Kraft with Lina Chan, Founder and CEO of Parla, a digital health platform putting data and knowledge back in women’s hands so they can be an informed agent in managing their own bodies proactively.
Can you describe in your own words what Parla does?
Parla is a digital health platform helping communities of women with specific fertility challenges connect with experts and each other to find the support they need.
Could you elaborate on how women connect with these experts and the communities?
There is no one-size fits all approach. The way women connect with their issues in fertility is inherently specific to their own pain points. It’s dependent on what the origin of their fertility struggles are. For example, many women with PCOS or Endometriosis end up struggling with their fertility. Other women may have faced multiple pregnancy losses during their fertility journey. What each woman needs can be very different and personal. As FemTech evolves – and we’ve already started to see that happen – there will inherently be more start-ups targeting very specific pain points (such as PCOS or Endometriosis-related infertility) because it connects with the user and their user journey a lot more.
Do you have specific fertility streams in Parla?
When we initially launched Parla we started it off as supporting women with any fertility struggle they had, but when COVID came we began to understand that our users bucket around certain pain points. One big category we have for example is pregnancy loss, another is PCOS and another major one is Endometriosis.
That’s why one of the main projects we launched last year are courses to specifically target how to manage the symptoms of these specific pain points. For example, for pregnancy loss how to manage the emotional distress that comes with pregnancy loss. We also have nutrition and lifestyle interventions to manage PCOS.
How do you see Parla personalizing women’s healthcare experience?
When women come to us we do a lengthy onboarding questionnaire so that afterwards the content they see is personalized to them. If we know the women faced pregnancy loss in the past then the they wind up seeing a lot more content around mental health support. A women with PCOS will get a lot more content around period health and lifestyle intervention for instance.
Why do you perform these interventions in a community format?
There’s an emerging body of research that supports grouping women in cohorts for long-term support. They learn just as much from each other as from the expert.
You wouldn’t believe the amount of ‘aha’ moments women have where they hear each other’s stories. They often think they are alone but then realise others have had similar experiences. With both our pregnancy loss courses and period health courses we ask women to make everyday changes to their lifestyle and the fact that they meet with experts and each other every week makes them much more likely to actually try the change and stick to it.
You mentioned you offer pregnancy loss courses. You are one of the few startups we came across that offer support for pregnancy loss. Why do you think there’s been such a lack of innovation in that respect when it’s so common?
Probably because it’s one of the biggest taboos we have in our society. I mean if you take a step back to untangle it: the twelve-week rule is crazy* (*The Twelve-week rule is the unwritten rule that says you should not share your pregnancy news until you hit the 12-week mark, the point at which most pregnancies are considered safe and likely to be successful).
Yes, that’s when the highest rates of miscarriage happen. But look at it from the reverse perspective, if someone has been taught to shut out their entire support system in the first twelve weeks that makes them even more vulnerable if they do miscarry because they can’t go to someone and tell them they’re struggling if they never told them they were pregnant in the first place.
We also just don’t talk about miscarriages. When you learn about Sex in Sex Ed we don’t talk about how precarious the first trimester is. We don’t say that one in four pregnancies end in miscarriage. This is not just a Femtech revolution – we need a societal revolution. This is a missed opportunity for innovation in women’s health because it has such an impact on physical and mental health. The medical community needs to realize this is a big issue that impacts women years later. No one ever prepares you to think about death when you are trying to conceive. But unfortunately the rates of pregnancy loss are high and increasing as women choose to have children later.
And there’s also a lot of misconception around pregnancy loss in general. We tend to think of it as being exclusively miscarriages but abortion is also pregnancy loss, having to terminate for medical reasons is also pregnancy loss, stillbirth is also pregnancy loss. It’s a risk category when it comes to mental health.
How do you see Parla evolving in the future?
When I launched Parla one of my main goals was to help women be more proactive about their health. As a platform we want to start flagging things that might be of consequence for the future. So even if we’re a fertility platform we start talking about things like menopause. We have information about the general stages they might be dealing with down the line. We start flagging other things they might want to look out for: from nutrition to lifestyle.
Reproductive health is so siloed in our society. And yet there are so many interdependencies: women who have issues with pregnancy and infertility tend to have poorer health and mental health outcomes later on. At Parla we firmly believe in the intersection of physical and mental health.
Innovations like Parla’s digital communities that help women feel less alone in their fertility journey are why we need Femtech. (And a FemTech Summit like this one of course). Want to hear more about how digitising the fertility journey could be key to providing more holistic care for women everywhere? Sign up for the 2022 femtech conference here.
__________________________________________________________________________________________________________________________________________________________________________________________________________________
 How Do Gendered Practices In Medicine Impact The Care Patients Receive ?
How Do Gendered Practices In Medicine Impact The Care Patients Receive ?
The following is a transcript of an interview by Oriana Kraft with Dr. Marjorie Jenkins, MD, MEdHP FACP, dean of UofSC School of Medicine Greenville, Chief Academic Office for Prisma Health-Upstate. This is Part 2 of a series of Deep Dives on Sex Specific Topics in Medicine, in the aim of connecting students with research being done in the field.
Could you tell me a little bit about how you came to be involved in sex and gender specific medicine?
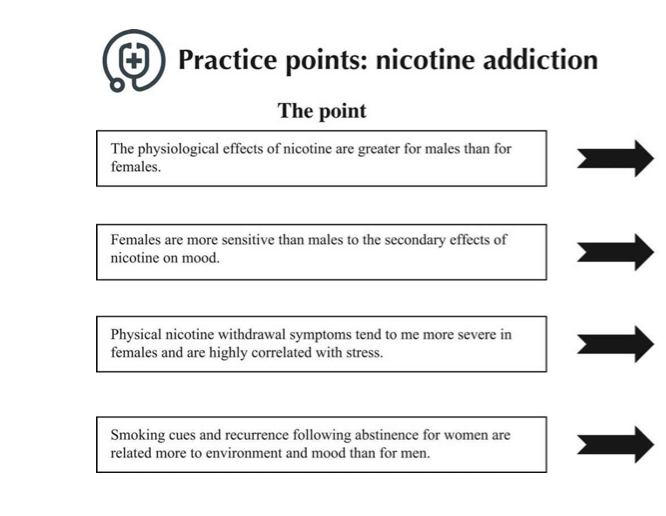
I became involved in the sex and gender specific medicine movement fairly early on – in 2002. Due to the NIH and the National Centers Of Excellence In Women’s Health programs, data on women’s health beyond just breast and pregnancy and lactation (so-called “bikini medicine) began in earnest in the late ‘90s. The Centers Of Excellence In Women’s Health were grant awarded to provide fund to academic health centers and community-based health centers for them to enhance their women’s health programs by conducting research into women’s health, develop training and educational programs or improve the sex specific clinical care they delivered. The program was defunded in 2005 but the work of the Centers of Excellence really started to get the ball rolling in terms of the collection of research data in both men and women for diseases like Alzheimer’s and cardiovascular disease.
Have we made progress in terms of the research we conduct on women’s health since 2005?
 Although more women are participating in clinical research, there are no requirements regarding the reporting of research data by sex and/or gender outcomes in most scientific journals. Even in basic science research, considering every single cell in the human body has a sex, it is still not a requirement in most journals to report the sex of the cells involved in the research studies. There is an NIH policy of Sex as a Biological Variable which was implemented in 2017. This policy required researchers to justify lack of inclusion of both sexes in research, if they are provided funding to research a disease that applies to both sexes and genders. However, this policy falls short in that it does not require analysis and reporting of outcomes by sex.
Although more women are participating in clinical research, there are no requirements regarding the reporting of research data by sex and/or gender outcomes in most scientific journals. Even in basic science research, considering every single cell in the human body has a sex, it is still not a requirement in most journals to report the sex of the cells involved in the research studies. There is an NIH policy of Sex as a Biological Variable which was implemented in 2017. This policy required researchers to justify lack of inclusion of both sexes in research, if they are provided funding to research a disease that applies to both sexes and genders. However, this policy falls short in that it does not require analysis and reporting of outcomes by sex.
What can doctors do to improve the care they deliver to their patients, given the biased nature of the research they are basing a lot of their practices upon?
Practicing clinicians need to avail themselves of the clinically relevant sex differences data that is already out there. There is a new textbook on clinically applicable sex differences which I edited with Dr Connie Newman. It is titled How Sex and Gender Impact Clinical Practice: An Evidence-based Guide to Patient Care, published by Elsevier in early 2020. It’s very case-based and applicable to primary care providers. We don’t have all of the answers regarding sex differences, but that doesn’t mean we don’t have a good amount of clinically meaningful data to improve the health of men and women already. Make no mistake: one-sex, gender-blind medicine isn’t just about women. Men are marginalized in some diseases processes and do not receive optimal care as well.
Could you give examples of conditions where men get poor care due to the gendered nature of the care we deliver? I would imagine it happens a lot in diseases like Alzheimer’s or autoimmune disorders…
Not Alzheimer’s. The majority of participants in Alzheimer’s studies are men, even though it eventually becomes a women’s health issue because women live longer with Alzheimer’s and are diagnosed later in life. Here are a few examples of how society often genders health conditions. When an elderly man comes into the emergency room and has fallen down and broken his wrist, emergency physicians will often send the patient home without an order for a scan for osteoporosis. If he fell down just walking across the yard, that’s a fragility fracture, and by definition he has clinical osteoporosis, so normally appropriate follow-up would be to prescribe a bone density scan for osteoporosis. And yet, less than 20% of men seen in the emergency room for fractures actually get the appropriate follow-up they deserve. Yet, men have a much higher likelihood of dying at one year after a fracture than women do. They are also much less likely to return home to independent living. We think of osteoporosis as your grandmother’s disease and this impacts the care men receive. If you look up osteoporosis online, the majority of images you receive are of women. In addition, the majority of bone density scans a located in breast imaging centers which further prevents men from seeking appropriate screening. So, men walk in, see all this information surrounding breast health and breast awareness and are given a pink slip to put on to in order to take the scan The second example where men are marginalized is urinary incontinence. It’s the number two reason why elderly men are institutionalized because their family cannot take care of them. And yet, guess where urinary incontinence pads are located in the neighborhood drug store? Next to the feminine products. This implicit bias dissuades men from purchasing the products they need. It’s all so gendered and marginalizes men’s healthcare.
 What about the reverse cases? What is an example, in your opinion, where the healthcare system is missing an opportunity to pursue gendered innovation?
What about the reverse cases? What is an example, in your opinion, where the healthcare system is missing an opportunity to pursue gendered innovation?
Pharmaceutical companies are definitely missing the boat. Women are much more likely to engage with the healthcare system, take medication and see physicians than men. And yet, the vast majority of medication we commonly use while tested in both sexes do not have the data analyzed by sex. This is much like we discussed in the earlier question. By not testing medication to see if it is more efficacious in women, pharmaceutical companies are losing the opportunity to market to half the global market. This as a real issue. It’s why, when women receive treatment they should ask if the treatment has been adequately studied for safety and efficacy in women. Women need to be their own advocates and begin expecting and demanding personalized treatment. They should ask questions like: “Has this been studied in women? What did the studies show? Is it more or less efficacious? Are there more side-effects?” Roughly 70% of drugs which can be used in women who are pregnant, or lactating have no human data in the drug label. That may seem difficult to believe, but in an audit of over 570 medications, this was born out to be true.
______________________________________________________________________________________________________________________
 This interview is part 1 of a series of Deep Dives on Sex Specific Topics in Medicine, in the aim of connecting students with research being done in the field.
This interview is part 1 of a series of Deep Dives on Sex Specific Topics in Medicine, in the aim of connecting students with research being done in the field.
How do hormonal transition periods impact female mental health?
The following is a transcript of an interview by Oriana Kraft with Claudia Barth, PhD, postdoctoral researcher at Diakonhjemmet Hospital and the Norwegian Centre for Mental Disorders Research, University of Oslo. Some of the questions and answers have been edited for brevity and clarity.
Hi Claudia! It’s so great to have you here – first off could you give a brief summary of the research you do?
I focus on how hormonal transition periods across the human lifespan impact the brain and mood in healthy women versus women with depression.
So you try to identify whether there are moments in time when women are particularly vulnerable to depression – could you elaborate on that a bit?
We know that women are particularly susceptible to depression during adolescence, post–partum and the transition to menopause when there are intense hormonal fluctuations– but what’s really interesting to study is why woman A develops post partum depression after giving birth, and not woman B? What role do hormones play in it? And what role do these normal hormonal fluctuations stemming from reproductive events play in brain ageing later in life?
Our research has shown that pregnancy, for instance, might have a positive impact on brain ageing. We are still trying to figure out why that might be exactly but we have a couple of hypotheses.
Do you have an example of a hypothesis?
They still need to be tested – but one potential hypothesis is that there are quite extensive changes in inflammatory profiles during pregnancy. For instance, there is an increase in regulatory T cells which suppress inflammation so that the fetus can exist. This upregulation of regulatory T cells may persist in women after birth, which can help to positively arrest immune responses when no longer needed. Ageing is associated with low grade inflammation. So if you have an upregulation of regulatory T cells that may suppress this mechanism, you can imagine that it could be beneficial for brain ageing later in life.
So if we know that women are particularly susceptible during hormonal transitions – do you have an example of how that might trickle down to clinical practice?
The knowledge that women are more vulnerable to mood disturbances during hormonal transitions has been out there for a while. So one easy way to account for it, would be to incorporate it into questionnaires. For instance, trying to ascertain whether a woman’s depressive symptoms are exacerbated pre-menstrually, can inform treatment decisions. Because what’s interesting about reproductive subtypes of depression is that they seem to be a stable trait. For instance, a woman with a history of premenstrual dysphoric disorder (PMDD, a severe form of premenstrual syndrome) has a greater risk for developing postpartum depression. Simply by knowing that, we can put frameworks in place to educate the partner andalert the midwife, in case this woman plans to get pregnant.
Another example would be that a rocky perimenopausal transition with depression, insomnia and night sweats might give rise to an accelerated neurological decline afterwards. So intervening earlier on, for instance with targeted hormonal therapy, might improve the woman’s outcome in the long term.
There is also evidence that commonly used antidepressants such as SSRIs (selective serotonin reuptake inhibitors) may work differently by reproductive stage. SSRIs are less effective during menopause, but may act faster in women with PMDD during reproductive years. So really just asking patientssex–specific questions can lead to better quality of care.
And you’ve started looking into how that can be done, right ?
Yes, I’m currently working with a local psychiatry clinic to incorporate pre-written, structured women-specific questions to better inform treatment decisions for women with depression . It’s an easy thing for a clinician to ask (among many other questions): “Do you use hormonal contraception?” or “Do you experience any physical or emotional symptoms before onset of menses?”. It is relevant information for assessing potential causes for depressive symptoms and subsequently for improving therapeutic approaches.
I mean, the funny thing when it comes to research is that everyone focuses on precision medicine. It’s such a hyped word, but then a lot of researchers don’t even stratify their study sample by sex. It is a long way to precision medicine if you don’t start there. It doesn’t mean that there are always sex differences between men and women in diseases. But certain conditions might have different implications for men or women. And if you don’t check for it – you’ll never know.
________________________________________________________________

The following is a transcript of an interview with Rachel Zsido, PhD student at the Max Planck Institute for Human Cognitive and Brain Science, currently studying sex hormones and how they influence brain structure, cognitive health and depression susceptibility.
Hi Rachel! Can you tell us a bit about yourself and your research interests?
My background is in neuroscience. I’m interested in how ovarian hormones and other biological factors interact to influence the brain, and what implications those interactions have for cognitive health and emotional wellbeing.
One of the studies I’m working on is ‘menstrual cycle plasticity’ . We’re looking at how subtle fluctuations in hormones across the menstrual cycle influence brain plasticity – specifically structural brain plasticity. We have women come in six times over the course of their menstrual cycle to assess how cycle-related fluctuations in hormones relate to brain volume, focusing on brain regions involved in emotion and cognitive processes.
We also have a premenstrual dysphoric disorder (PMDD) project, where we look at how ovarian hormones influence the serotonergic system across the menstrual cycle in PMDD patients. For context: we know that 800 million people menstruate daily, of which roughly 75% of them will experience premenstrual symptoms. Roughly 8% of menstruating women will experience the more severe form, PMDD, which is characterized by severe symptoms of anxiety, depressed mood, mood swings and irritability.
As the symptoms occur exclusively in the late luteal phase right before menstruation, and only during ovulatory cycles where we have these fluctuations in hormones, the immediate thought might be that there is a hormonal difference in these women. But a decent amount of research has shown that there’s no difference in absolute levels of hormones in women with and without PMDD.
So it seems that, although hormones are clearly involved, women suffering from PMDD have a sensitivity to normal cyclical, hormonal changes rather than abnormal hormone levels. That’s why PMDD is now discussed as a reproductive subtype of depression. It’s not the same as major depressive disorder. The symptoms may overlap but are not the same – the most important aspect being that the symptoms are phase-locked.
From a clinical perspective, we do know that antidepressants that slow the reuptake of serotonin, such as selective serotonin reuptake inhibitors (SSRIs), vastly improve symptoms within hours to days for patients with PMDD, compared to patients with major depressive disorder or anxiety disorder where antidepressants typically take two to three weeks to alleviate symptoms. That, alongside the important role of serotonin in mood regulation, suggests that PMDD symptoms may be driven by a hormone-induced change in serotonergic function – this is what we’re looking into.
And the last study examines inflammatory and immune marker patterns across the menstrual cycle to look at the cyclical nature of female immunity, which could have really important implications for healthcare. If you know how inflammatory patterns vary cyclically, maybe medical intervention timing could be optimized by cycle phase, especially when treating inflammation-related disorders. Maybe a better understanding of menstrual cycle regulation of the immune system could inform your decision of when to make a vaccination appointment. And perhaps more related to my field, if your menstrual cycle information can be used to calculate inflammatory load over the lifetime, this could influence the way we think about brain aging and risk trajectories over the female lifespan.
How do you see FemTech intersecting with research?
I love that FemTech not only focuses on new software and technologies for improving women’s healthcare but is also providing ways to close the data gap – we’re finally collecting datasets that address uniquely female experiences, such as the menstrual cycle, postpartum, and menopause transition. FemTech is simultaneously improving healthcare standards for women as well as advancing research on people typically excluded from studies.
Neuroscience is notably guilty of this systematic bias, with the male brain often implicitly treated as the default model. As I study susceptibility and resilience to depression, cognitive decline and dementia, this still shocks me. We know that women make up 2/3 of Alzheimer’s disease patients and are twice as likely to suffer from a depressive illness. So the population that is at greatest risk for these disorders is actually understudied, and we’re not talking about a small group of individuals – we’re talking about over half the world’s population. I think FemTech and research can intersect by combining interactive digital health technology and personalized healthcare options with neuroscience and clinical research to determine neuropsychiatric and neurodegenerative disease risk over the female lifespan, and then to optimize medical intervention timing and development.
Do you have an example of how FemTech could be used to improve healthcare?
For PMDD, aside from a medical history and psychological interviews, there are currently very few diagnostic tests and treatment options. A lot of women use birth control or antidepressants to help with PMDD symptoms. But these may not be ideal options for many women, given that symptoms only occur a few days out of the month, and these medications are really designed to be taken chronically. FemTech is already helping by providing better ways to track your menstrual cycle and record any related changes in mood and body. By tracking your cycle, you start to see the patterns, you gain control, you may be able to predict upcoming changes in mood, and you can almost ‘biohack’ the menstrual cycle. It’s just more data! You can walk into your doctor’s office and show them data.
Also our hormones are so much more than just our reproductive organs! Hormones influence the brain, our mood, our cognition, cardiovascular health… the list goes on. I think FemTech has the capacity to connect our reproductive health to full body health. So beyond developing medical intervention on a largescale, I love the idea that, by integrating in an individual’s own data, you can create personalized programs for improving one’s own health.
FemTech could also be used to leverage information from one hormonal transition period to inform later hormonal transition periods in life. Back to the PMDD example that we’ve talked about earlier – studies have shown that you have an increased risk of experiencing postpartum depression if you experienced PMDD earlier in life. So this sensitivity to the drop in hormones that occurs at the end of the menstrual cycle might be informative for more pronounced shifts in hormones later in life, such as during the postpartum period or perimenopause.
If you think about that in terms of synergies with FemTech, it could be particularly powerful if we developed technologies to build longitudinal datasets for monitoring reproductive health measures over the lifespan, to be used to better understand potential risks trajectories for brain health or whatever you’re interested in. It could inform preventative care and prepare women for upcoming changes in the body and brain in a proactive, de-stigmatizing fashion.
I’m really interested in brain aging in particular – because brain aging is the result of little steps that you take across your whole lifespan. If we could develop a tool that would allow you to conveniently record and assess the impact of all these little steps on your brain – in the context of accessible reproductive health measures – I think that would be really interesting.
________________________________________________
The following is a transcript of an interview by Oriana Kraft with Chloe Bird, PhD, senior sociologist at RAND.
Chloe Bird served as senior advisor in the National Institutes of Health’s Office for Research on Women’s Health and editor-in-chief of Women’s Health Issues, where she is now associate editor.
 Could you briefly describe the work you do?
Could you briefly describe the work you do?
I study sex and gender differences in health and healthcare as well as women’s health. I look at differences in the determinants of health and analyze how you get exacerbations between biologically and socially created differences. I look at quality of care in healthcare delivery and how we can make it better. Part of my work involves looking at female specific problems and the lower level of attention that they get in most cases and how those are impacted by biases in the science.
By female specific problems what do you mean exactly?
Health conditions that only affect biological female bodies such as certain reproductive cancers as well as those which disproportionately affect women like rheumatoid arthritis, breast cancer or osteoporosis. Those are all very useful cases for studying what the implications of being an understudied group are. We don’t have any good interventions for men who develop osteoporosis. Developing severe osteoporosis puts you at risk of a hip fracture that is likely to be immediately or long-term fatal.
WHAM (Women’s Health Access Matters) published a report on “The case to fund women’s health research” that showed that if we were to invest only $6 million in rheumatoid arthritis research we would get $10.5 billion in returns to our economy. If we invest $20 million in coronary artery disease research focused on women, we would get $2 billion in returns to our economy.
Those numbers are shocking on their own but I’m curious, why do you think the return on investment is so much larger for rheumatoid arthritis when compared to coronary artery disease?
The return on investment is larger in rheumatoid arthritis than coronary artery disease because rheumatoid arthritis is more heavily female and has an earlier onset than cardiovascular disease. Women tend to be over sixty-five when they experience a cardiovascular incident, so it has a smaller impact on the years they’re able to spend in the workforce. Rheumatoid arthritis tends to manifest when women are in their forties or early fifties which can be debilitating and take them out of the workforce for many years.
When you skew what you study and prioritize, relative to the actual burden of the disease, you end up not optimizing what you could be doing in terms of reducing disease and disability. Part of it is we initially prioritized studying life-threatening diseases in men. And then overfit our understanding of those diseases (like coronary artery disease) for what they looked like on men – without understanding very well the way they work in women. As a consequence, our measures of understanding, of diagnosis and treatment – all of our research is on the basis of what we’ve observed and studied in men. But that means we end up missing and not dealing with a lot of the issues that are unique to women. It’s as if you had a Venn diagram. A portion of what we know from our studies on men can definitely be applied to women – but the rest of the circle that doesn’t overlap with men? That’s largely or completely uncharted territory.


Could you elaborate a bit on why that might be?
The gold standard for studying health is a clinical trial. You want a homogenous enough population where it’s a simple enough condition that you know you’re acting on disease A and not disease B, C, D or E instead. So we tend to study people who only have one condition and aren’t more complicated. So clinical trials skew a little bit younger within the population with the disease. So even if you’re just analyzing what kind of men we’ve actually researched you end up with a Venn diagram between the men you studied and what the male population in the real world looks like who actually have the disease.
But the overlap in the Venn diagram is much, much smaller for women. Because women tend to have multiple comorbidities (much more so than men) which means that what you find out from the clinical trial is much less applicable to women. The consequences for treating the world that actually experiences the disease, the entire population, is different because of the comorbidities, the polypharmacy, the need to integrate information from other specialties because many medications don’t just impact one organ or disease. So if we started studying women, what we’ve done for men in terms of studying them in clinical trials – which we are a long, long way away from – that won’t be good enough. The entire population is different because of the comorbidities, the polypharmacy, the need to integrate information from different specialties because of the ways things interact.
It makes total sense when you explain it that way but for a clinical trial you want to isolate and distill what you are studying to the smallest possible variable – so how can we go about fixing the way clinical trials are conducted for them to better reflect women’s medical situations? It seems unlikely that we’ll start studying multiple variables at once even though the medical reality is that women face multiple issues at once.
If we went at it from the perspective of ‘what have we already answered, what is it we know, what is it we don’t know’. If we actually did a topography of the burden of disease and mapped onto it ‘what have we already asked, where have we gotten answers?’ We could start to look at where we haven’t looked. We could start to look at what we need to do to improve population health and that alone could use objective data. That would start to tell us where there are opportunities that we haven’t pursued. Part of what I’m pushing for is the science hasn’t been objective. It isn’t objective. By failing to be responsible for looking at it is that we don’t know we are falling short.
We have these series of assumptions in science. For example, RAND did a systematic review for the Veterans administration. They were interested in knowing: ‘Is there evidence for evidence-based treatment for alcohol and drug abuse disorders?’ When I read the report, it talked about there not being any evidence of sex and gender differences – but it was written up wrong, or at least incompletely.

How so?
Well, the report said there was no evidence of evidence-based treatment for alcohol and drug abuse disorders between men and women – but that wasn’t the case. The reality was no one asked. There was no evidence for evidence-based treatment because no one conducted studies on the topic.
The systematic ‘not having looked’ is a big problem in science. It influences how we categorize what we know. Logical decisions dictated that we simplify science. But because of hubris we decided that what we learnt from our studies on men was information we could generalize for women, even though we know we got different results in women. We just decided that we got different results because of the menstrual cycle or later onset of disease.
Since that time, we have much more evidence that the differences are due to differences in biology down to the cellular level. We have much more evidence that there are sex and gender differences but we are still conducting our studies in the same way: as if we can apply what we learnt in men to women. Which is a justification that is purely based on agreement not on evidence.
I’m working on a piece on the statistical models we use in medicine. In statistics, we generally say : “here’s how complex the problem is. In all the cases that are simplified to this standard we can do this much simpler statistics. But in order to use simplified statistical models you need to show results that say that your data fits that assumption for the simplified model and acknowledges it’s an assumption. But we haven’t put that standard in place for statistical models in medicine that says: “do you have any evidence that the underlying correlation matrix for male-bodied people and female-bodied female is actually similar enough to justify the use the admitted oversimplification of applying what we learn in men directly to women?” We could raise the bar on science by doing science as science is intended instead of following the familiar short-cuts by saying they’re good.
________________________________________________________________________
That’s why we need Femtech. And FemTech Summit’s like this one. Want to hear more about why investing in women’s health just makes economic sense? Sign up for the 2022 femtech conference here.

What would happen if we decided that the assumptions we made about medical practices could not be justifiably generalised to women. Would it cause us to throw out a lot of the medicine we use for women – if it’s all based on this assumption that was never proven?
I’m not arguing that we throw it out. I’m arguing that we study how useful is this? We don’t know how much of our evidence generalizes. And we should know that. If we are interested in studying human health. We actually need to study humans and the diverse populations they are composed of. We routinely generalize beyond data. After studying ten thousand people – we generalize the results of the trial for beyond that. We still act as if females are just smaller males. We treat the organs in a women’s body as if they’re just plug and play. We treat pregnancy the same way when, in fact, it’s a window into cardiovascular disease.
What do you mean?
You can think of pregnancy as a nine-month stress test on the cardiovascular system.
During pregnancy a woman might get gestational diabetes or gestational hypertension. These are likely to resolve after pregnancy when you don’t have the additional stress of the fetus in your system – and so it’s often treated as a resolved issue once the woman has given birth but it’s a missed opportunity. It would be an opportunity for us to have window to be able to look at how the woman is doing. The vast majority of women with gestational diabetes go on to develop diabetes eight years later. That’s a missed opportunity. We could have used when the woman got it during her pregnancy as a treatable moment.
Are you saying we don’t routinely include the fact that a woman developed gestational diabetes during her pregnancy when we look at a woman’s long-term risk of developing cardiovascular disease?
No – reproductive health is completely siloed from the rest of healthcare. The woman is seen over in obstetrics and then it’s treated as if you went and did this thing and now it’s over. It’s like you ran a marathon and that was that. You’re back to being ‘normal’ again and we’re not going to incorporate it in your patient file. Although we could and should flag it and track it. It’s a highly teachable moment. When you’re younger you have an immediate desire to be present and healthy for ten, twenty years for your child. So one possibility is while you have this person with a concern for how am I going to make this all work and stay around and be healthy. One possibility is that you help make the case that it’s going to be more important in your life to incorporate these behavioral changes. It would be consistent with the ways we do other types of medicine.
Why do we think of cardiovascular disease as a male disease?
Men were dying of heart attacks. That’s what was visible. That was the concern. There were odd theories like if women went into the workforce they would have heart attacks like men. So they defined the case on the visible part of the problem. They defined the diagnostic approach, the levels of intervention but that doesn’t mean that’s the disease that just means that’s what you see. We did the same thing for ADHD. It wasn’t that girls didn’t have the problem it was just that it wasn’t as visible. They didn’t act out in the same way. It’s the same thing with men and heart attacks. We saw men dying of heart attacks so that’s what we built the model on. The problem is: women have far, far higher rates of ‘silent’ heart-attacks. They’ve usually sought care a lot of times before their heart attack and it’s dismissed – because our diagnostic measures don’t reflect the way women’s heart attacks manifest.
As a result, medicine is a house on stilts. There are a few foundational pieces that we’re right on but beyond that we don’t know. And the problem is not that it’s a house on stilts but that it’s a house on stilts pretending it’s not a house on stilts. Which means we never do the hard work of evaluating what we actually know objectively and what we don’t.
We’ll learn a lot more by studying diverse populations. If you believe that it’s always been okay that we studied men, that it was good enough, this has worked – not a problem. Let’s just take thirty years – study women and generalize to men – while we get more information, because we will learn more by studying women than men, and even it out. If you have a problem with that answer than your first response wasn’t accurate.
I don’t think it’s necessary or desirable to stop studying men. I think we just need to do complete work in analyzing the data. For example, offering on-site child care benefits women a lot more than men. It can impact breastfeeding. It can impact a woman’s experience of parenting because of the oxytocin or her risk of breast cancer. It’s not going to do that for men. It’s a social intervention. But we need to understand that our lives accumulate and build differences that are not inherent or inherited. They’re created and they always will be.
Healthcare in the US was designed for catastrophic care. It covers catastrophic care much better than comorbidities and chronic diseases. But women have more comorbidities and doctors’ visits. But because we set it up to treat everybody the same, it costs women a lot more out of pocket. We created a formula that rests on the assumption that men and women are the same, even though women’s circumstances, health and life expectancy aren’t the same. Our definition of what is equal and fair is way more expensive for women.
I fundamentally believe that we will do a better job of validating the science that we have by studying the populations that we haven’t studied. Shockingly that means studying the majority group: women. And that’s where breakthrough science is going to happen. We are going to learn more about being human by studying populations that we haven’t studied and recognizing where we made assumptions and shortcuts.
The following was a continuation of the interview Oriana Kraft conducted with Chloe Bird, PhD, senior sociologist at RAND.
Chloe Bird served as senior advisor in the National Institutes of Health’s Office for Research on Women’s Health and editor-in-chief of Women’s Health Issues, where she is now associate editor.
We wanted to close off this interview with a stat: Did you know that investing 26 million dollars in women’s health adds back nearly 40 000 years of full-time employment for both women and men? 40 000 years – can you imagine that? That’s why we need femtech to shine the spotlight on the fact that we need more women’s health research (and a femtech summit like this one to showcase the innovation that’s happening in this burgeoning space). Want to hear more about why investing in women’s health just makes economic sense? Sign up for the Summit here.